So Does Protein Cause Insulin Resistance?
Hold on a minute. Did you say protein can cause insulin resistance?
Last week on X, whilst participating in an open discussion around protein intakes and the downsides of eating too much protein, I pointed out that there is a growing body of evidence that high protein intake in someone who is overweight can acutely cause insulin resistance and keep them obese.
What surprised me was that the conversation was with several people who are very intentional about their health, including a PhD.
I don't know if this PhD was having a bad day, but me suggesting this seemed to really trigger him. He was not interested in seeing any of the evidence I was referring to and simply told me I was lying.
In the last Uncivilized post, I briefly explored some of the high-level mechanistic evidence for this link between protein and insulin resistance.
Some of those studies were done decades ago. So I wanted to do a full post exploring what really is the modern association between these two things and any conclusions we can draw from it.
Fundamentally, at a high level, the critical thing that we need to understand is that people suffering from obesity are in a protein-sparing mode.
They are carrying abundant additional energy stores, and at Patchwork we line them up so that their body can to tap into those excess energy stores and not catabolize any protein or muscle as you lose weight.
This also means that they do not need to be eating one gram of protein per pound of body weight, especially as we don't recommend people suffering from obesity for doing excessive exercise, thus lowering protein needs further.
Insulin resistance is a condition where cells in the body's muscles, fat, and liver don't respond effectively to insulin.
If you want to go down a 🐰 hole on all the chronic illnesses which stem from insulin resistance. This is a great book.
So Does Protein Cause Insulin Resistance?
The relationship between protein intake and insulin resistance has been a topic of significant debate within metabolic research.
While protein, particularly in the form of branched-chain amino acids (BCAAs), is essential for numerous physiological processes, recent studies suggest it may play a nuanced role in metabolic dysfunction.
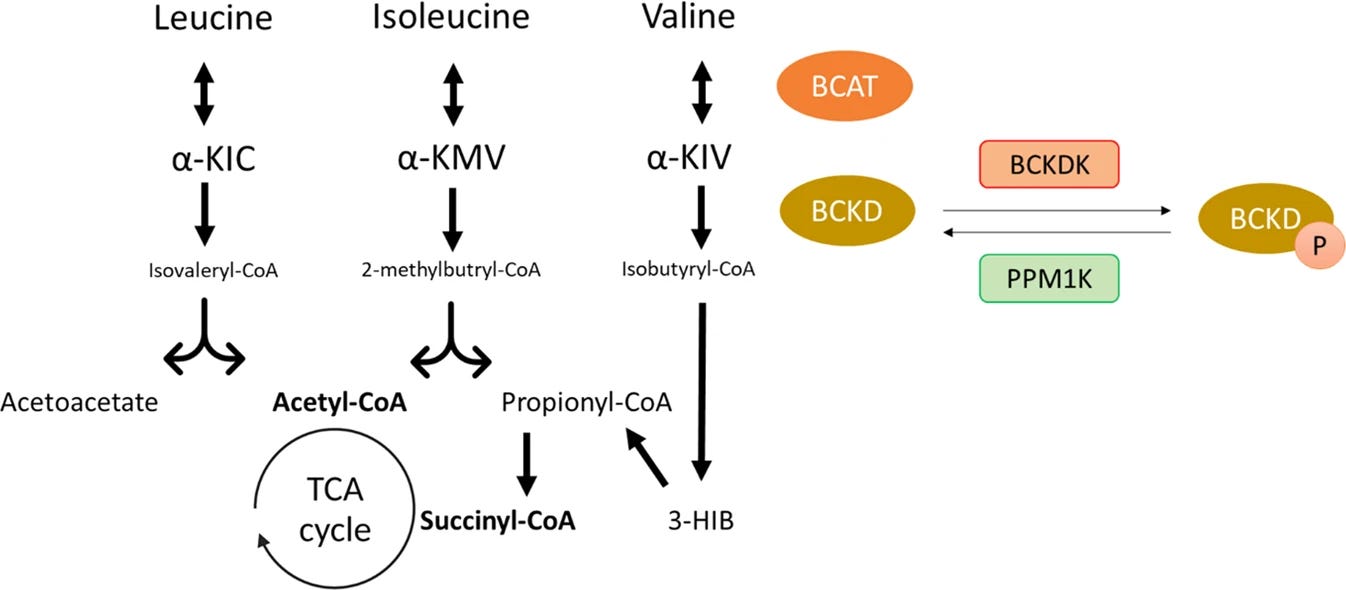
Insulin resistance, a hallmark of type 2 diabetes and obesity, appears to be influenced by dietary protein composition and metabolic pathways involving amino acids such as leucine, isoleucine, and valine.
Early research demonstrated that elevated plasma amino acid levels, especially BCAAs, were correlated with hyperinsulinemia in individuals with obesity.
This finding highlighted the potential for protein-derived amino acids to modulate insulin secretion and sensitivity.
Subsequent studies have expanded on this by examining the role of BCAA metabolism in the etiology of insulin resistance. For instance, this study underscored that impaired BCAA catabolism in obesity could lead to toxic accumulation of BCAA metabolites, exacerbating insulin resistance and systemic inflammation.
Mechanistically, BCAAs have been shown to influence key metabolic pathways that drive insulin signalling.
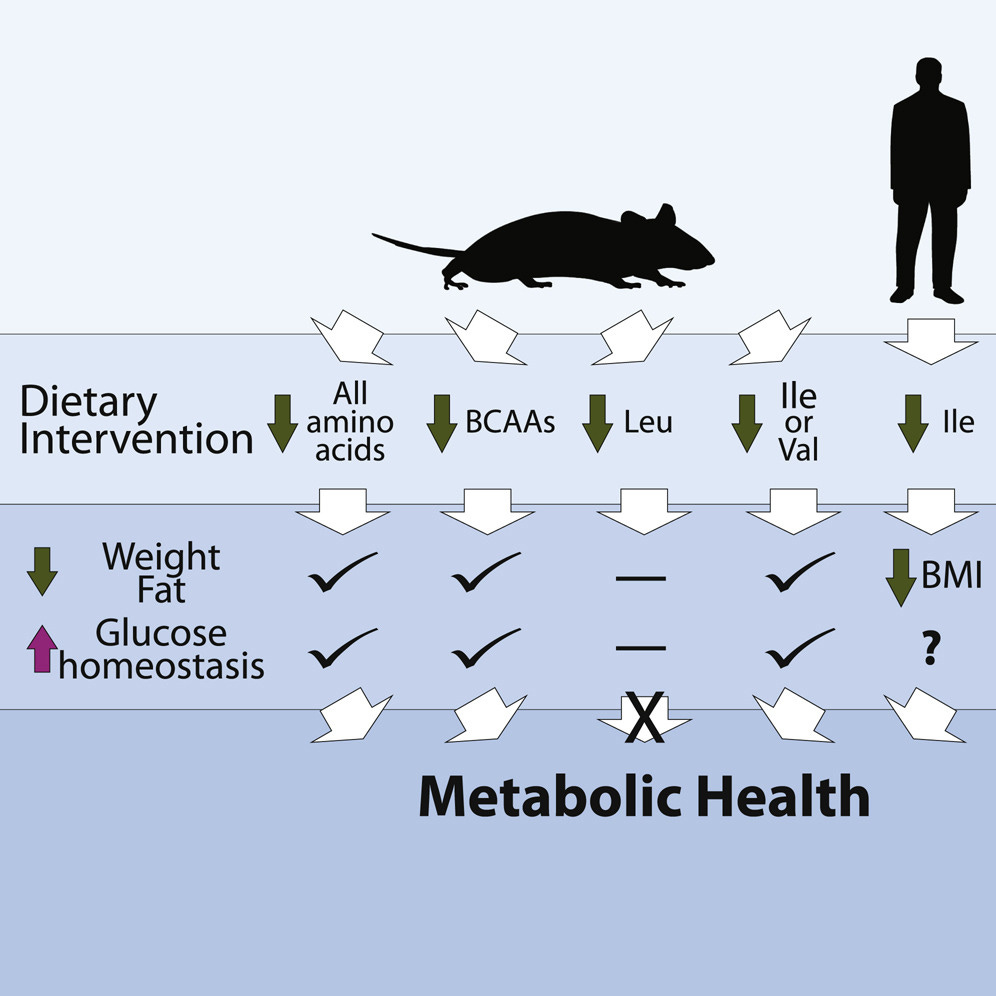
A study in 2016 revealed that a BCAA-derived metabolite, 3-hydroxy-isobutyrate, promotes vascular fatty acid transport, linking BCAA metabolism to ectopic lipid accumulation—a critical factor in insulin resistance. Furthermore, Fontana et al. (2016) demonstrated that dietary restriction of BCAAs could ameliorate metabolic health, improving insulin sensitivity and glucose tolerance.
The adverse metabolic effects of excessive protein intake, particularly from diets high in animal protein, have also been highlighted.
Kevin Hall observed that ketogenic diets, often rich in animal protein, increase circulating BCAAs and lipid intermediates, which can impair insulin signaling.
Similarly, this study in 2021 identified isoleucine and valine as the primary mediators of BCAA-induced insulin resistance, implicating these amino acids in the worsening of metabolic health.
But, not all studies support a direct causal role for dietary protein in insulin resistance. Solon-Biet argued that the effects of protein on insulin sensitivity depend on the balance of dietary macronutrients.
The insulin resistance research suggested that protein-induced insulin resistance may be mitigated by reducing overall caloric intake or by achieving a more favorable balance between protein and carbohydrate consumption. This highlights the complexity of dietary interactions and the insulin resistance influence on metabolism.
On a molecular level, excessive dietary protein may lead to nutrient signalling dysregulation via the mTOR pathway, a key regulator of cell growth and metabolism.
Back in 2014 Lynch and Adams emphasized that chronic mTOR activation by BCAAs might desensitize insulin receptors, impairing glucose uptake.
This aligns with the findings of Ramzan, who demonstrated that reducing BCAA levels through dietary intervention improved metabolic parameters in individuals at risk of type 2 diabetes.
Interestingly, plant-based protein sources, which are typically lower in BCAAs, appear to have a more favorable effect on insulin sensitivity.
This study in 2022 noted that diets emphasizing plant proteins might reduce systemic BCAA levels and improve metabolic health. This aligns with growing evidence supporting the role of plant-based diets in mitigating insulin resistance and associated metabolic disorders.
The role of protein in insulin resistance may also vary depending on the metabolic context.
Bloomgarden highlighted in 2018 that while high-protein diets might exacerbate insulin resistance in individuals with preexisting metabolic dysfunction, they can also promote weight loss and improve glycemic control in certain populations.
This superposition underscores the need for personalized dietary recommendations based on individual metabolic profiles.
In conclusion
While protein itself does not universally cause insulin resistance, excessive intake of BCAAs and animal-derived protein, particularly in the context of obesity and high-fat diets, may contribute to metabolic issues.
As covered in my last post about protein needs - high protein diets are generally great for athletes and lean aging populations who are concerned about sarcopenia.
But if you are overweight, massive animal protein intakes can be one of the things blocking your fat loss and gumming up your metabolism.
Finally - I am not here to take away your meat or keto diets. I love meat. Hell I buy a cow every year and this year slaughtered my own thanksgiving turkey. I’m just saying there are some things to consider here - More Protein does not equal More Fat Loss.
Bibliography
Bloomgarden, Z. (2018). Diabetes and branched-chain amino acids: What is the link? Journal of Diabetes, 10(5), 350–352. https://doi.org/10.1111/1753-0407.12645
De Bandt, J.-P., Coumoul, X., & Barouki, R. (2022). Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity. Nutrients, 15(1), 68. https://doi.org/10.3390/nu15010068
Felig, P., Marliss, E., & Cahill, G. F. (1969). Plasma Amino Acid Levels and Insulin Secretion in Obesity. New England Journal of Medicine, 281(15), 811–816. https://doi.org/10.1056/NEJM196910092811503
Fontana, L., et al. (2016). Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell Reports, 16(2), 520–530. https://doi.org/10.1016/j.celrep.2016.05.092
Hall, K. D., et al. (2021). Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nature Medicine, 27(2), 344–353. https://doi.org/10.1038/s41591-020-01209-1
Jang, C., et al. (2016). A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nature Medicine, 22(4), 421–426. https://doi.org/10.1038/nm.4057
Lynch, C. J., & Adams, S. H. (2014). Branched-chain amino acids in metabolic signalling and insulin resistance. Nature Reviews Endocrinology, 10(12), 723–736. https://doi.org/10.1038/nrendo.2014.171
Ramzan, I., et al. (2020). A Novel Dietary Intervention Reduces CInsulin resistanceculatory Branched-Chain Amino Acids by 50%: A Pilot Study of Relevance for Obesity and Diabetes. Nutrients, 13(1), 95. https://doi.org/10.3390/nu13010095
Solon-Biet, S. M., et al. (2019). Branched chain amino acids impact health and lifespan indInsulin resistanceectly via amino acid balance and appetite control. Nature Metabolism, 1(5), 532–545. https://doi.org/10.1038/s42255-019-0059-2
Vanweert, F., Schrauwen, P., & Phielix, E. (2022). Role of branched-chain amino acid metabolism in the pathogenesis of obesity and type 2 diabetes-related metabolic disturbances. Nutrition & Diabetes, 12(1), 35. https://doi.org/10.1038/s41387-022-00213-3
White, P. J., et al. (2021). Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Molecular Metabolism, 52, 101261. https://doi.org/10.1016/j.molmet.2021.101261
Yu, D., et al. (2021). The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metabolism, 33(5), 905-922.e6.https://doi.org/10.1016/j.cmet.2021.03.025







My own N=1
I did the lion diet for about 3 months my starting weight was 180lb
I ate beef to satiety for 2 months which ended up being 3-4 pounds of chuck beef so more than 200g of protein a day. I didnt eat butter or excess fat on top of that.
I GAINED about 12 pounds during that time but i lost a lot of viceral fat (waist 37 inch -> 34 inch)
Id share any bloodwork if i had any but i didnt do any during that period.
I also got pregnant for the first time in my life, realizing my copper IUD had been displaced for the past 8 years. Doing absolutely nothing... The entire time... I ended the diet due to that but in the end I lost the pregnancy in the first trimester.
So i would say that "too much" protein definitely can cause weight gain and exasperate obesity. but given my overall health and fertility improved during that period, im still on the side of "to satiety" carnivore diet being on the "good" end. Still, some form of restriction on top of that is a requirement for weight loss.